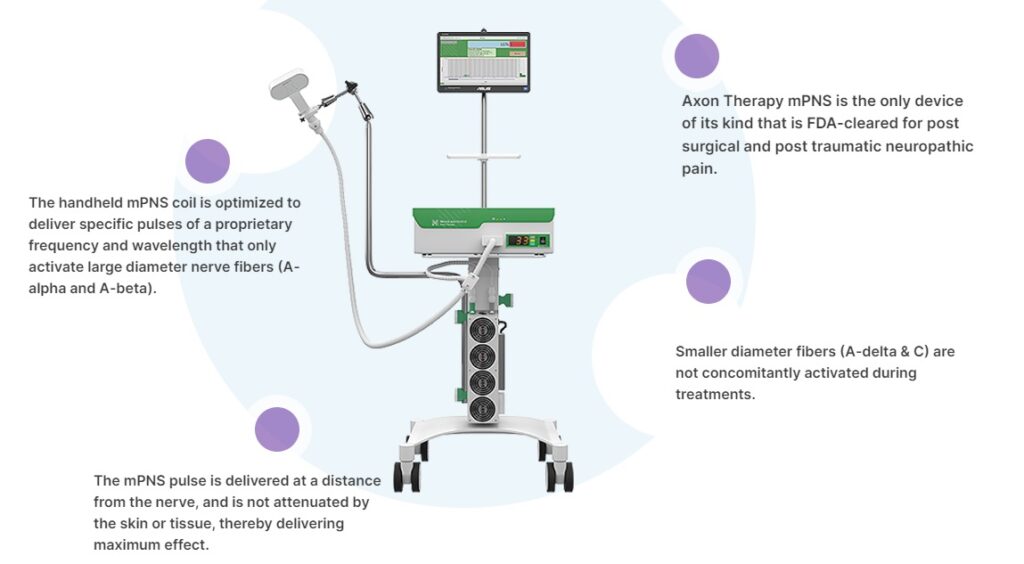
San Diego–based Neuralace says this marks the first-ever FDA clearance of a non-invasive, magnetic peripheral nerve stimulation (mPNS) treatment for PDN. The company says its Axon Therapy could offer “new hope” to millions with the condition.
Axon Therapy uses mPNS to deliver a quick, painless and non-invasive treatment in sessions lasting just 13.5 minutes, according to Neuralace. Each session utilizes magnetic pulses to provide relief for a potential improvement in pain management.
Neuralace said a recent trial of 71 patients demonstrated efficiency, plus significant improvements in subject outcomes. The company believes its therapy represents a paradigm shift in PDN treatment.