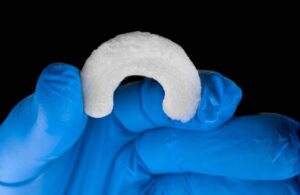
The minimally invasive, collagen-meniscal implant aids in the reinforcement and repair of soft tissue injuries of the meniscus. RejuvaKnee delivers a more complete recovery by facilitating the regeneration of the native meniscal tissue instead of cutting or replacing it.
In a 12-month animal study, RejuvaKnee demonstrated that, in three months, its regenerated meniscus can withstand full weight bearing. Additionally, the knee returns to the normal range of motion. The company says its results prove superior to those of meniscectomy and allograft transplantation.