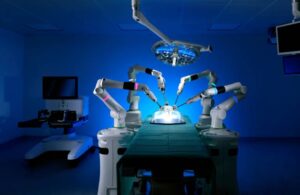
Cambridge, UK-based CMR says clearance paves the way to introduce a next-generation, adaptable, digitally driven surgical robot in the U.S. The company says Versius becomes the first multi-port, soft tissue general robotic-assisted surgical device to gain clearance through the FDA’s de novo process.
Versius delivers assistance in the precise and accurate control of Versius Surgical endoscopic instruments. Its indication covers adult patients (22 years of age and older) eligible for soft tissue minimal access surgery for cholecystectomy.