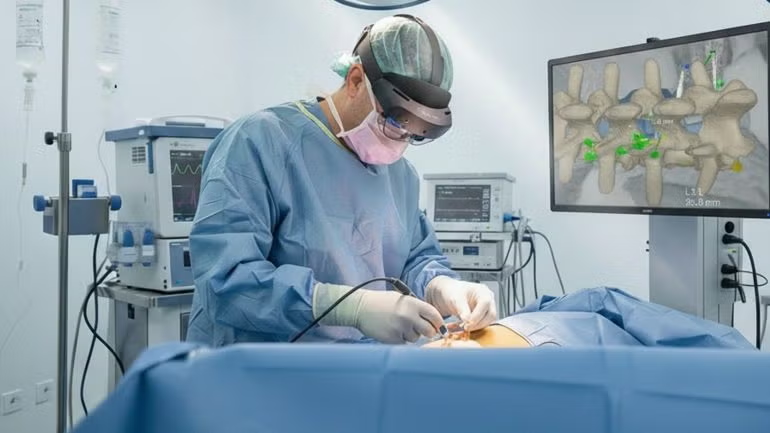
The US Food and Drug Administration (FDA) has granted 510(k) clearance to Surgical Theater’s next-generation platform, SyncAR Spine.
The decision enhances the platform’s suite of extended reality (XR) tools, which are underpinned by AI algorithms.
SyncAR Spine enables the direct incorporation of computed tomography scans and magnetic resonance imaging (MRI) into the surgical environment, facilitating a transition from preoperative planning to intraoperative execution.