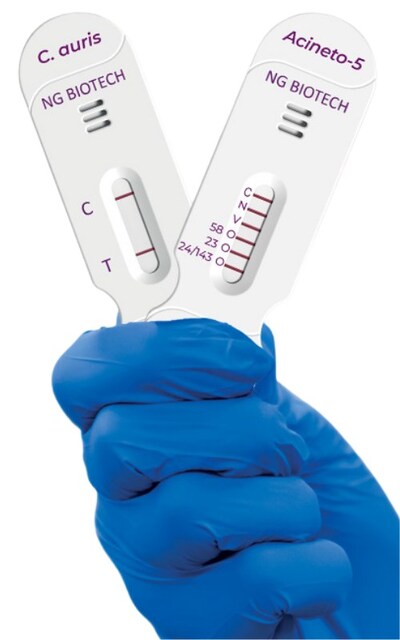
Both tests target pathogens classified as critical priorities by the World Health Organization (WHO). Candida auris, listed in the WHO Fungal Priority Pathogens List (2022), is a multidrug-resistant yeast responsible for hospital outbreaks worldwide. It is often difficult to detect and associated with high mortality. Carbapenem-resistant Acinetobacter baumannii (CRAB), included in the WHO Bacterial Priority Pathogens List (2024), is among the most dangerous hospital-acquired bacteria due to its resistance profile and rapid transmission in healthcare settings.
NG-TEST® Candida auris is the first rapid lateral flow immunoassay specifically designed to identify C. auris from cultured samples in 15 minutes. Published data demonstrate 100% concordance with reference methods across diverse isolates, supporting its role in outbreak investigation and infection control.