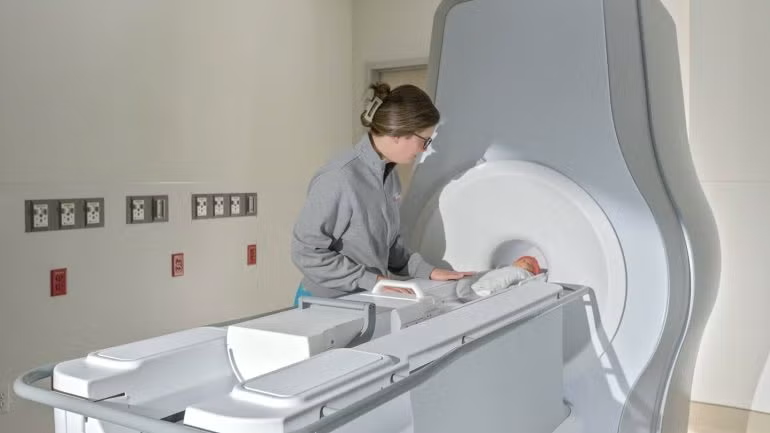
The US Food and Drug Administration (FDA) has granted 510(k) clearance for Eyas Medical Imaging’s Ascent³ᵀ neonatal magnetic resonance imaging (MRI) system, a device engineered specifically for the anatomy of neonates and infants.
The Ascent³ᵀ is said to be the first high-field, three Tesla (3T) MRI system dedicated to neonatal and infant imaging.
It features a 3T magnet that enables comprehensive and detailed scans of vital organs such as the heart, lungs, abdomen, and brain.