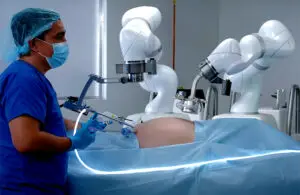
The FDA granted 510(k) clearance for its Magnetic Surgical System (MSS), combined with MARS, in bariatric and hiatal hernia repair. Mountain View, California-based Levita says the expanded indication reinforces its ability to support surgeons treating patients with obesity-related conditions. It provides a less invasive solution that enables simultaneous treatment of hiatal hernias during bariatric surgery.
FDA clearance enables clinicians to use the MARS system’s Dynamic Magnetic Positioning technology. This retracts the liver and enhances visibility and access during hiatal hernia repairs. Clearance offers an innovative, less invasive option for abdominal surgery and paves the way for broader adoption of the technology, Levita said