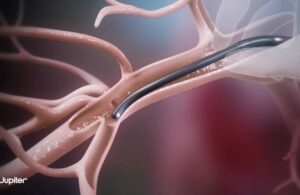
The company can now conduct the SPIRARE II U.S. pivotal study for the pulmonary embolectomy system. Menlo Park, California-based Jupiter Endovascular only just came out of stealth mode, spinning out of Neptune Medical this month.
Vertex incorporates Jupiter’s novel Endoportal Control platform technology into an endovascular procedure. It designed the technology to treat acute pulmonary embolism (PE) with control and precision.
The company developed Endoportal Control technology to bring these benefits to a variety of catheter interventions. Jupiter Endovascular aims to enable interventionalists to deliver treatment to anatomical sites that can’t be safely or easily reached through conventional endovascular approaches.