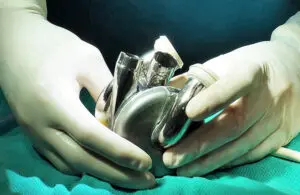
Five patients received the BiVacor TAH between July and November 2024 in the study looking at the TAH as a bridge to donor heart transplant. The initial phase supported patients with the TAH system for up to a month while they waited for a donor heart. These patients had severe biventricular failure.
After the five successful implants and subsequent discharges, the FDA had enough data to greenlight the expansion of the EFS. This allows BiVacor to expand its trial to an additional 15 patients.