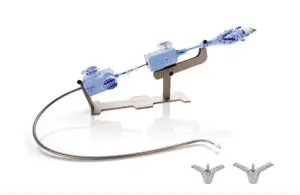
The Circulatory System Devices Panel of the Medical Devices Advisory Committee for the FDA confirmed this with votes registering 13 to 1 in favor of TriClip’s benefits. Abbott submitted TriClip, a transcatheter edge-to-edge repair (TEER), to the FDA for premarket approval in March 2023. The company expects a decision on that submission from the FDA this year. The FDA will consider the panel’s vote when making its final decision.
If approved — which could prove more likely with the panel’s affirmative vote — it would take on the recently approved Edwards Evoque tricuspid valve, which became the first transcatheter therapy approved to treat TR earlier this month. While they both treat TR, Abbott labeled TriClip a first-of-its-kind minimally invasive device. It specifically designed TriClip to treat the difficult-to-access tricuspid valve.
Analysts previously said they expect approval for TriClip, which received its most recent CE mark in 2021. It holds approval in more than 50 countries.