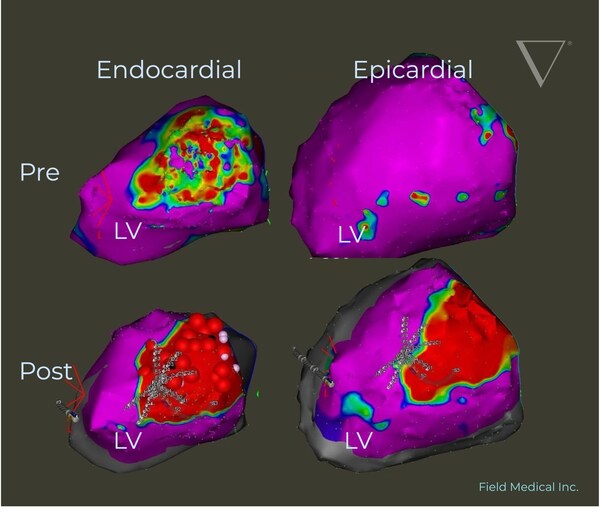
CARDIFF-BY-THE-SEA, Calif., Dec. 5, 2024 /PRNewswire/ — Field Medical® Inc., a leader in pulsed field cardiac catheter ablation technology, announced today that its FieldForce™ Ablation System has been accepted into the FDA’s Total Product Life Cycle Advisory Program (TAP) Pilot and granted Breakthrough Device Designation for sustained monomorphic scar-related ventricular tachycardia (VT). This recognition underscores the system’s groundbreaking approach to addressing life-threatening VT and the critical unmet needs for patients worldwide.
“The FDA’s TAP Pilot acceptance and Breakthrough Device Designation for the FieldForce™ Ablation System represent pivotal milestones in our journey to regulatory approval,” said Dr. Steven Mickelsen, CEO of Field Medical. “This recognition advances our vision of equipping electrophysiologists with a next-generation, focal PFA tool for fast, accessible VT care.”
Dr. Vivek Reddy, Director of Electrophysiology at Mount Sinai Health System, added, “The FDA’s recognition of this breakthrough technology underscores the urgent need for innovation in treating complex, life-threatening ventricular tachycardia. Field Medical’s ablation system has the potential to redefine VT care for physicians and patients alike.”