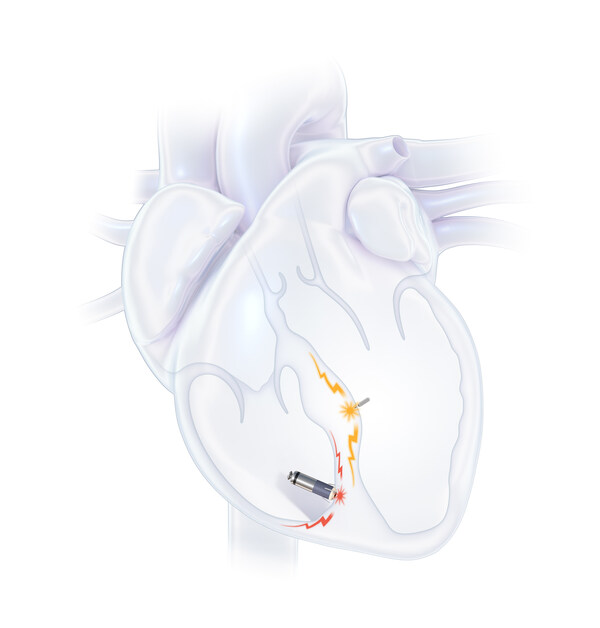
The WiSE System is the first and only leadless device to deliver LVEP—offering a more physiological1 approach that mimics the heart’s natural electrical activation by stimulating the endocardial tissue inside the left ventricle. This expands treatment options for heart failure patients who have exhausted conventional therapies.
“Treating our first commercial patient was a powerful moment,” said Dr. Robert Canby.
“We can now offer a leadless LVEP option for patients who were either unable to receive left ventricular pacing or whose prior therapies failed. Delivering pacing without navigating the coronary sinus is a major advancement. We’re excited to continue building experience with the WiSE technology.”
The procedure took place at St. David’s Medical Center of Austin in Texas, one of several leading institutions participating in the initial limited market release of the WiSE System.