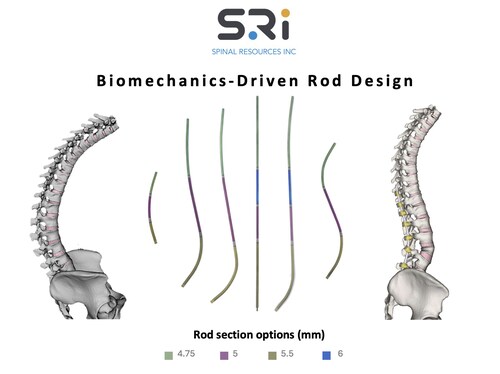
By allowing this first-of-its-kind universal clearance, the FDA has taken an important step toward acknowledging the sophistication of both the design and compatibility of SRI’s Bezier Parametric Curve Spinal Rod System, as well as enhancing the reach and adoption of truly innovative independent technologies for spine surgeons seeking options for their patients.
Dr. Saman Shabani, MD, the Director of Spinal Oncology Surgery, Adult Spinal Deformity Surgery and the Spine Fellowship Program at the Medical College of Wisconsin, had this to say about the clearance, “From a surgeon perspective, the universal clearance for the Bezier Rod system is ground-breaking because it gives me clinical liberty to achieve the best possible outcomes with the best possible technology without being restricted to a single supplier or device manufacturer construct.”