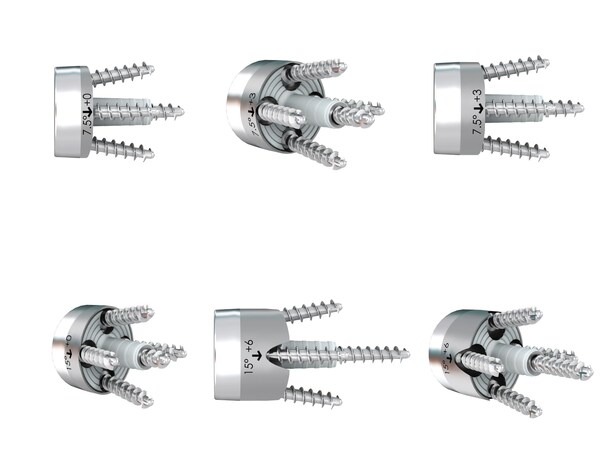
The glenoid baseplates are all 24mm in diameter with full-wedge options at 7.5° and 15° that have options to lateralize 0, +3, or +6mm. Each glenoid baseplate has four peripheral screw holes that have 12° of polyaxial variability that can be fixed with 4.5mm standard or locking screws. The option to use a 4.5mm central screw can be utilized through the central post for additional fixation (7 central screw length options from 8-20mm in 2mm increments). All glenoid baseplates are made from titanium (Ti6AV) and have a hydroxyapatite coating (CP Ti/HAP).
“This clearance of the full-wedge augmented baseplates further solidifies our portfolio as one of the most comprehensive and innovative shoulder arthroplasty platforms on the market,” said Baptiste Martin, CEO of FX Shoulder Solutions.