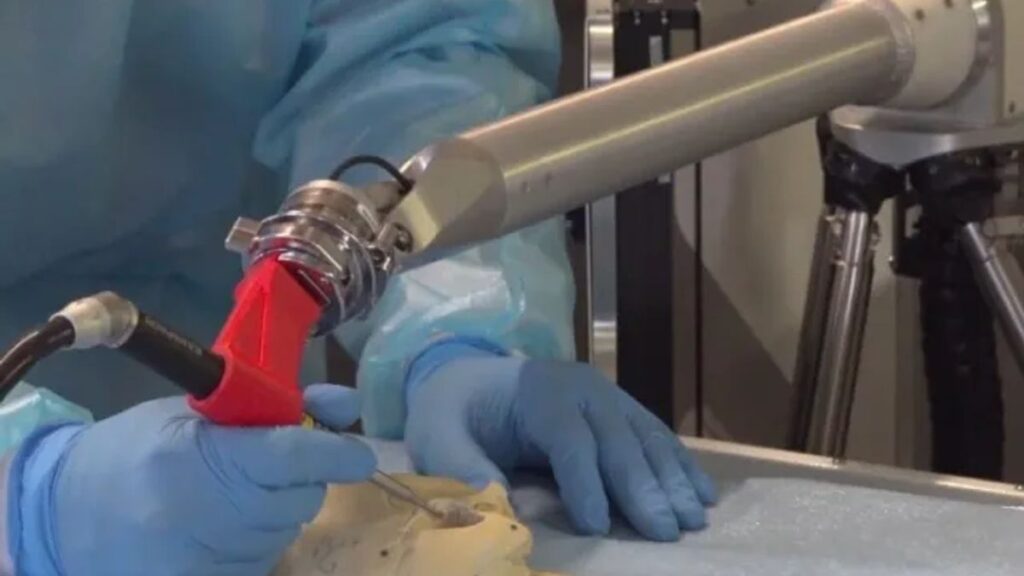
Galen Robotics, a startup founded in 2016 to commercialize the work of a lab at Johns Hopkins University, said its surgical robot has received the Food and Drug Administration’s de novo authorization for ear, nose and throat procedures.
The company also said it is opening a bridge round to its series B financing. It plans to use the funds to secure materials and expand manufacturing capabilities to support its growth plans.
Beyond ENT procedures, Galen’s platform is being developed to fill “unserved needs” in the surgical device market to assist surgeons in specialties including neurosurgery, otolaryngology, tissue reconstruction, intrauterine brain and spine surgery, according to the company.