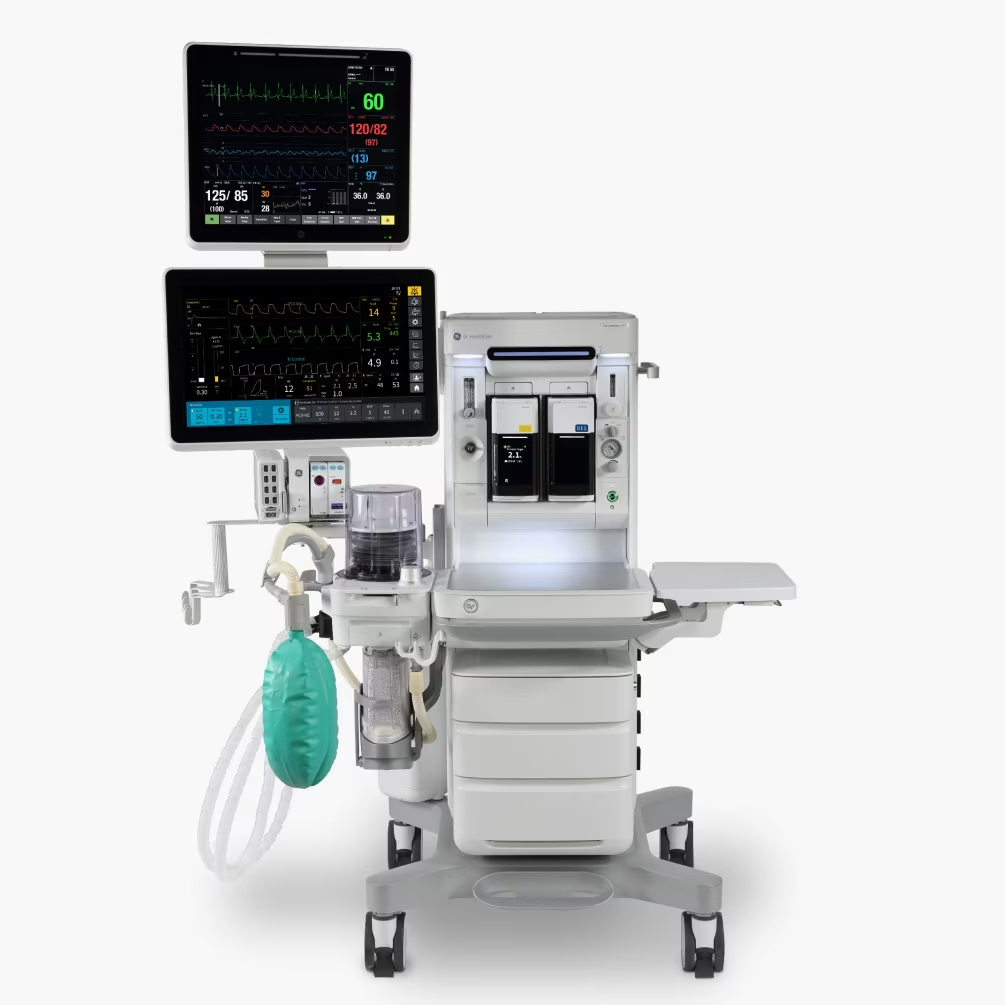
The FDA 510(k)-pending system aims to help care teams adapt to evolving clinical and operational needs. It could potentially advance anesthesia care through tools, customizable applications and a sleek, space-conscious footprint.
Chicago-based GE HealthCare’s FDA submission follows recent CE mark in Europe and approval in Australia and New Zealand.
Dr. John Beard, an anesthesiologist, serves as chief medical officer for Patient Care Solutions at GE HealthCare. Beard said in a news release that the new system can help ease burdens and support safer, more effective care delivery.