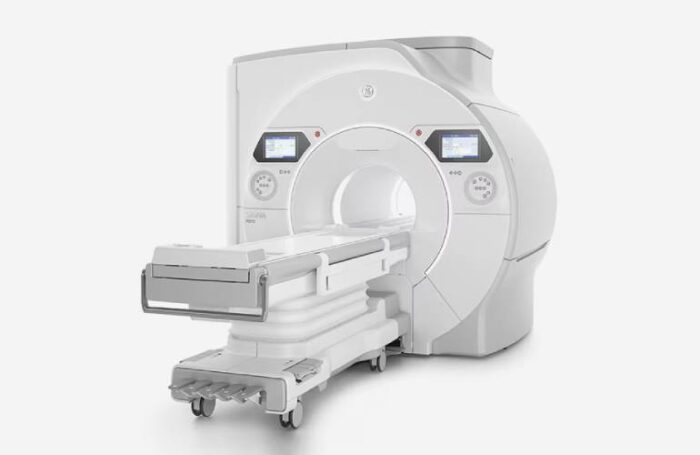
The FDA cleared the Signa Sprint with Freelium, Signa Bolt and Signa One. Signa Sprint is a 1.5T sealed magnet MRI system, while Signa Bolt is an advanced 3T MRI scanner. Signa One offers an AI-driven ecosystem of workflow solutions designed to help reduce inefficiencies and support MRI exams from plan to scan and beyond.
Altogether, the company says its new MRI systems and technology combat some of the most complex challenges facing the radiology landscape.