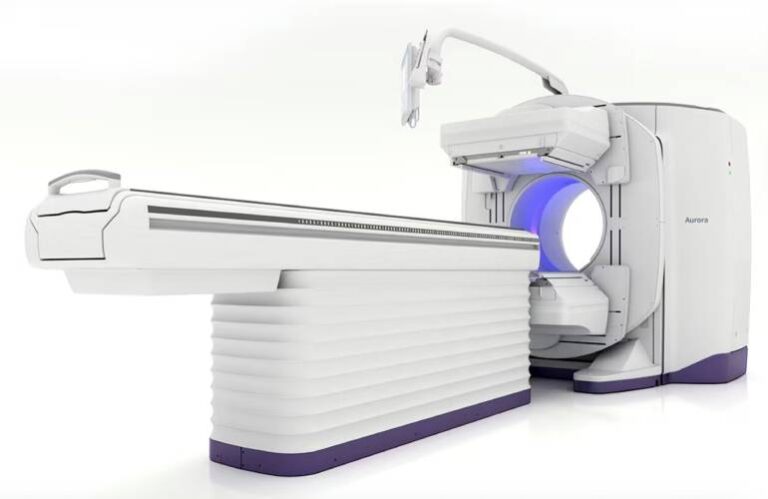
The company designed its advanced, dual-head SPECT/CT technology to enhance diagnostic capabilities and streamline workflows. It offers clinicians high-quality imaging and operational efficiency.
GE HealthCare says that clinicians now require more advanced SPECT/CT solutions providing greater diagnostic accuracy and efficiency. Monitoring and tailoring treatments to individual patients depends on high-quality imaging that can detect subtle disease markers.
To meet evolving healthcare needs, the company said it aims for Aurora and Clarify DL to seamlessly integrate into various clinical settings. This ensures that hospitals and imaging centers can adopt them without disrupting existing workflows.