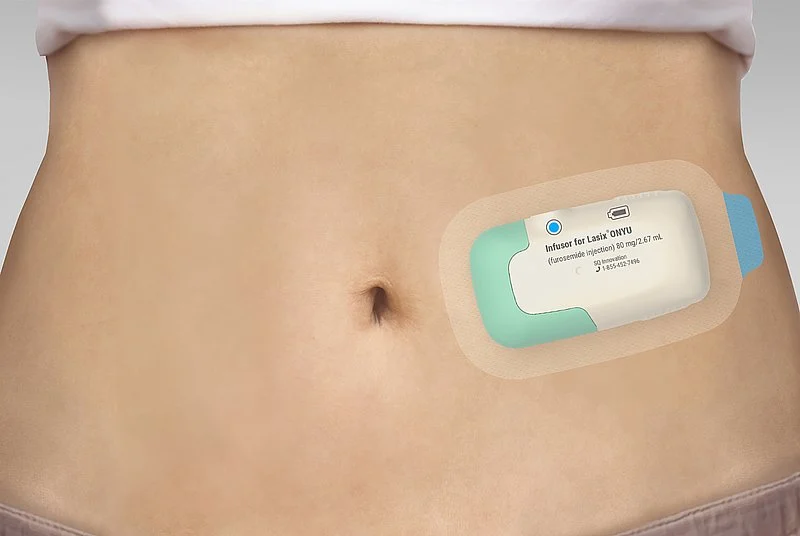
Lasix ONYU is a combination product consisting of a novel high-concentration formulation of the diuretic furosemide and the Gerresheimer on-body drug delivery device. Tentative Approval indicates here that Lasix ONYU has met the regulatory standards for quality, safety and efficacy required for approval in the United States. Full approval was precluded because the FDA had granted market exclusivity in the USA for a competing product until October 2025. SQ Innovation will seek full approval in the U.S. after the expiration of the regulatory exclusivity period. First products of Lasix ONYU are now expected to be available on the market by the end of 2025. The Tentative Approval of the combination product underscores Gerresheimer’s innovative strength and the market readiness of the Gerresheimer on-body drug delivery device.
“The FDA’s Tentative Approval is a testament to our product and the people and partners who have contributed to this great endeavor, especially the Gerresheimer team”, says Pieter Muntendam, MD, Founder, President and CEO of SQ Innovation. “It is an important milestone. We look forward to commercializing this highly innovative combination product as soon as we receive final approval with the aim to improve patients’ quality of life and reduce healthcare costs for the elderly.”