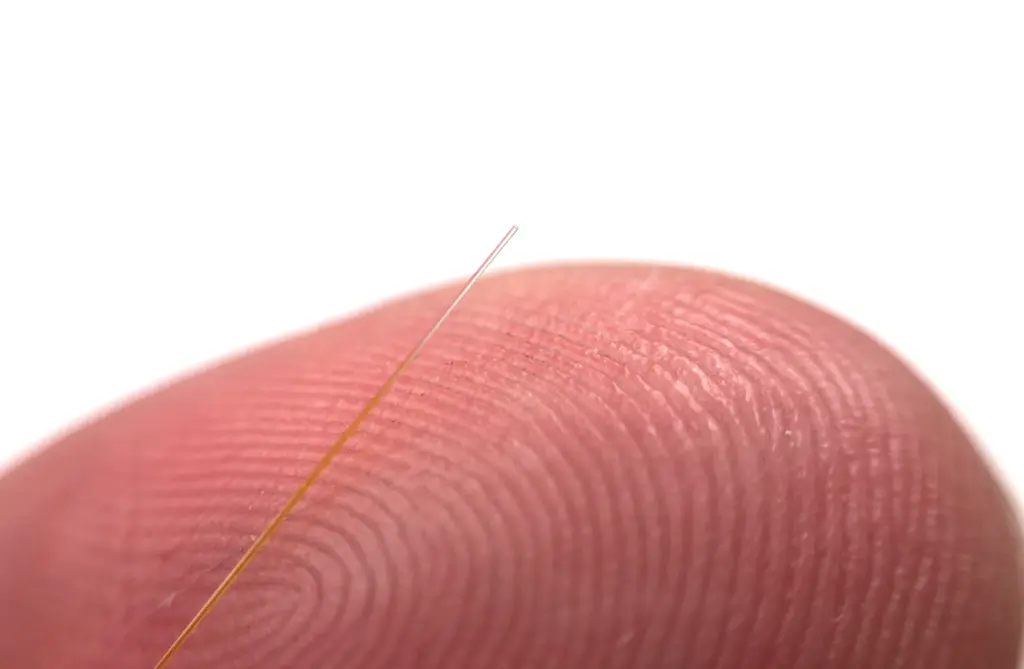
The company took to LinkedIn to share: “GlucoSet just received breakthrough device designation from the FDA! We’ve been pursuing this for a year and a half. Landing it puts us on the fastest possible path to U.S. revenue.”
Norway-based GlucoSet developed its biosensor system with a hydrogel matrix incorporated with a glucose-binding molecule. The gel contracts at rising glucose concentrations as a consequence of glucose-induced cross-binding of the molecules. Its hydrogel is fabricated at the tip of an opticla fiber and the whole sensor is covered witha semipermeable coating.
Changes in glucose concentration cause the volume of the gel — measured by an interferometric technique — to change, causing its diameter to change accordingly.