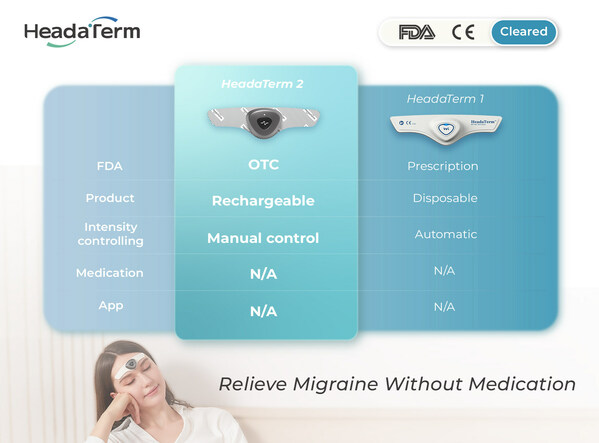
VANCOUVER, BC, April 2, 2024 /PRNewswire/ — WAT Medical Enterprise achieves a major milestone: HeadaTerm 2 receives the OTC clearance from the U.S. Food and Drug Administration (FDA), making it one of the only wearable anti-migraine devices that is available without a prescription in the U.S.
Using neuromodulation technology, HeadaTerm 2 releases targeted electrical impulses to increase pain tolerance of its users. It is FDA-cleared for the preventative treatment of migraine headaches.
The device has been clinically proven to be safe and effective at reducing migraine symptoms; according to the study published in the American Journal of Emergency Medicine, it can be up to 27%[1] more effective at relieving migraine symptoms than oral medications. Additional clinical studies featuring HeadaTerm 2 will commence in 2024 at world-renowned research institutions including University of Toronto, McMaster University, and Ohio State University.