With clearance, the Mountain View, California-based company says its algorithm is now available in the U.S. It also has coverage from Cigna across all of its lines of business beginning in October. Cigna joins UnitedHealthcare in providing coverage for the plaque analysis tool.
It marks the latest positive development for Heartflow after the company last month closed an upsized initial public offering (IPO) with proceeds totaling approximately $364.2 million.
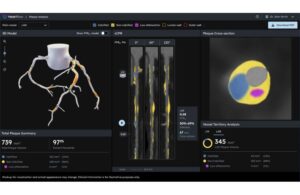
The technology features an updated algorithm, expanded nomogram and advanced, 3D color-coded visualization of plaque type, volume and distribution. Heartflow says it empowers clinicians with the insights needed to make confident care decisions with ease. The company touts it as the only FDA-cleared, AI-powered plaque quantification tool. This approval comes nearly three years after the platform’s initial nod from the FDA.