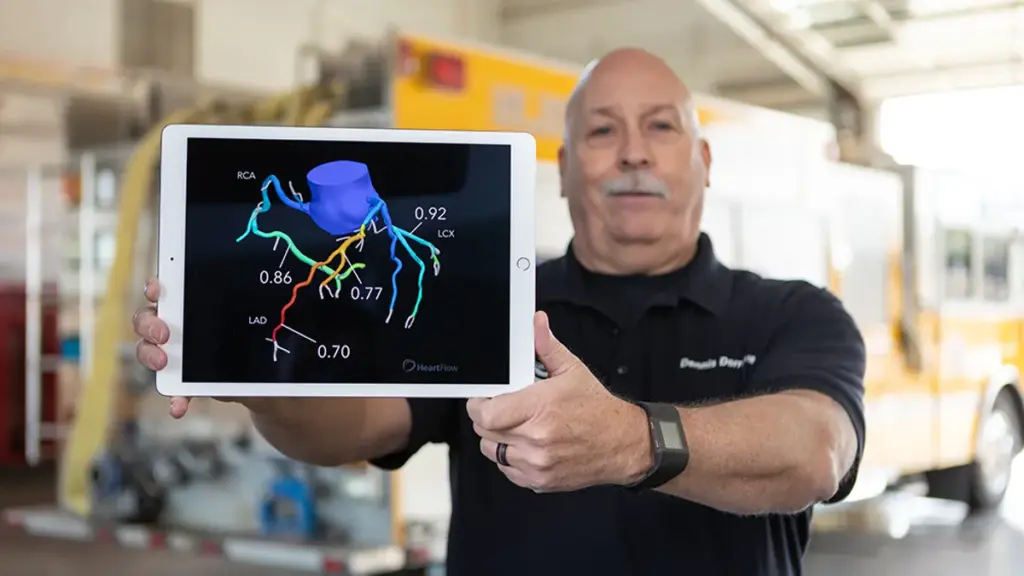
Using artificial intelligence, the product analyzes coronary CT angiograms, or CCTAs, to find and quantify plaque and thereby support the optimization of medical treatment strategies. A registry study linked treatment changes informed by the algorithm to reductions in LDL cholesterol.
Heartflow received Food and Drug Administration clearance for the first version of the product in 2022. The company began limited market education in the second half of 2023 but Heartflow FFRCT Analysis, its first product, still accounted for 98% of total revenue as of June 30. Heartflow has identified the ability to drive adoption of the plaque analysis product as a key factor that will affect its performance.
The clearance of the updated device gives Heartflow new features to emphasize as it seeks to grow sales of the plaque analysis product. Heartflow said the newly cleared technology has an updated algorithm and 3D color-coded visualization of plaque type, volume and distribution. The product is underpinned by data on 273,000 patients.