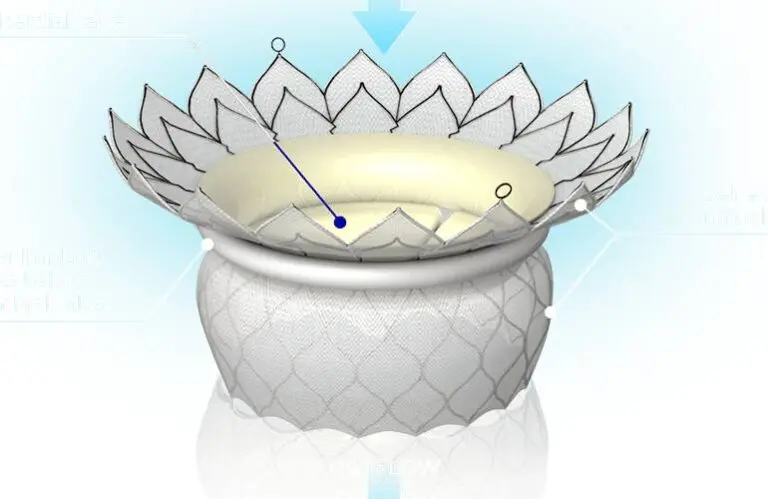
Paris-based HighLife — which has facilities in Irvine, California — develops a novel transcatheter system for treating mitral regurgitation (MR). It features a valve-in-ring concept, with both the ring and valve implanted percutaneously. Through a simple, three-step procedure, the valve goes into a beating heart, reducing trauma to patients. The company says it addresses the limited options for patients at high surgical risks, as TMVR delivers a less invasive alternative to open-heart surgery.
The MR treatment market is currently led by Abbott and its MitraClip transcatheter mitral valve repair system. Edwards made a major TMVR play last summer with the $300 million acquisition of Innovalve. Capstan, 4C Medical, InnovHeart and more are vying for a share, too.
HighLife’s valve remains investigational, under evaluation in clinical studies across three continents. FDA breakthrough designation enables the acceleration of the development and review of the TMVR system. It also provides frequent interactions with the FDA to facilitate a faster path to approval.