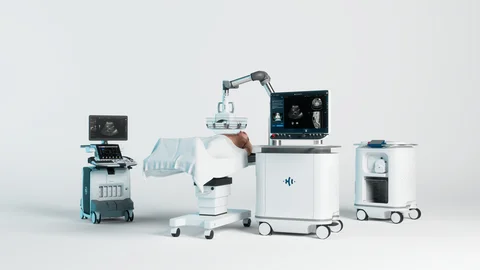
HistoSonics’ recent achievement in expanding insurance coverage for its Edison® Histotripsy System marks a pivotal advancement in non-invasive cancer care. With Highmark Blue Cross Blue Shield issuing four new positive medical policy decisions, approximately 7 million members across New York, Delaware, Pennsylvania, and West Virginia now have access to this FDA-cleared liver tumor treatment. This expansion builds on the system’s October 2023 De Novo clearance from the U.S. FDA, which recognized the Edison System for the mechanical destruction of liver tumors using focused ultrasound—a significant regulatory milestone validating its clinical potential and safety.
The Edison System employs histotripsy, a novel technique that uses focused ultrasound energy to mechanically liquefy targeted tissue without the need for incisions, radiation, or systemic toxicity. This method offers significant advantages over traditional therapies by minimizing complications and recovery time. Clinical data, including results from the HOPE4LIVER trial, have shown promising outcomes, with a 90% local tumor control rate at 12 months. Such performance not only supports clinical adoption but also strengthens payer confidence, encouraging broader integration of histotripsy as a frontline therapy in oncology centers.