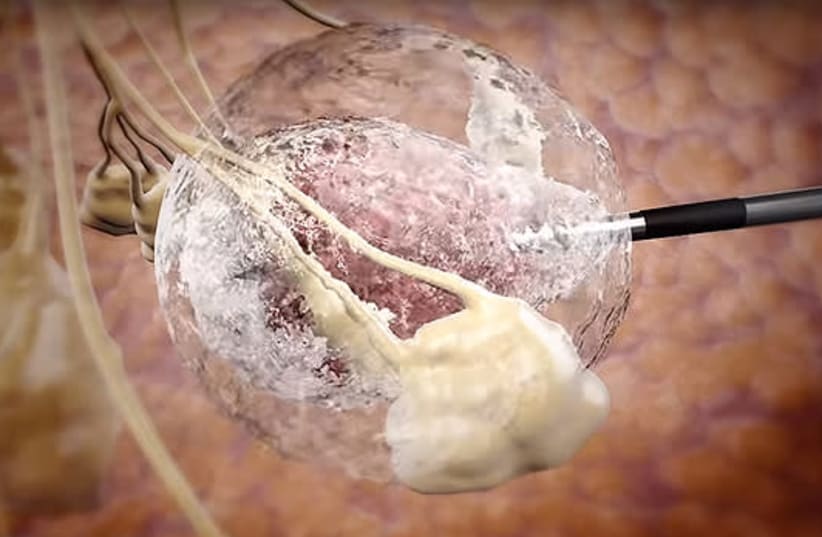
The notice covers the company’s invention titled “Cryogenic System Connector,” which recently received a patent in the U.S., too. Caesarea, Israel-based IceCure designed it to work as part of its XSense cryoablation system. The connector aims to improve safety and maintain the integrity of cryogen used in the cryoablation procedure.
IceCure designed the XSense system to destroy tissue by applying extreme cold temperatures. Targets include fibroadenomas, kidney tissue, liver metastases, tumors, skin lesions and warts. XSense received FDA clearance in July 2024 for all indications for which the company’s ProSense system already holds FDA clearance. That includes minimally invasive cryoablation in general surgery, dermatology and neurology, including cryoanalgesia. It also covers thoracic surgery, ENT, gynecology, oncology, proctology and urology.
The system utilizes liquid nitrogen, creating large lethal zones for maximum efficacy in tumor destruction. IceCure says the system accelerates recovery while reducing pain, surgical risks and complications. It also has an easy, transportable design for fast, convenient, office-based procedures