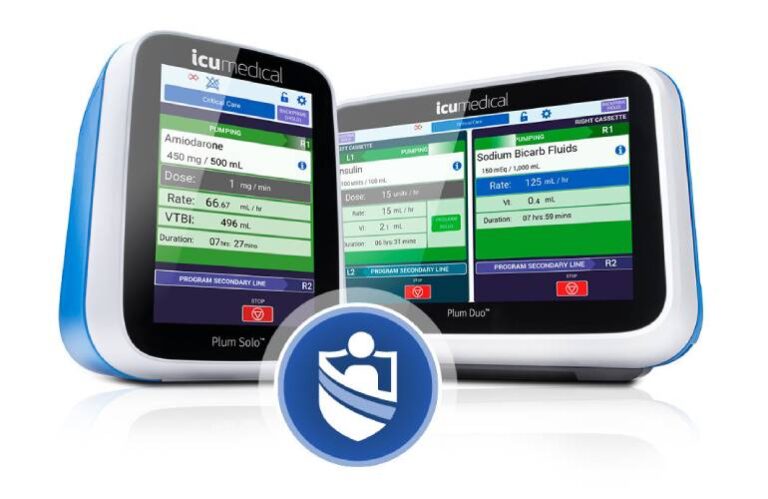
The FDA cleared the Plum Solo precision IV pump, a single-channel complement to the dual-channel Plum Duo. ICU Medical also got the FDA’s green light for updated versions of its Plum Duo pump and LifeShield infusion safety software. This completes the initial launch of the company’s IV Performance Platform.
With the clearances, ICU Medical said it’s introducing a new category of precision IV pumps and expanding its IV Performance Platform.
ICU Medical built its precision pumps to address delivery variability while supporting the increasing need for accurate data. Plum Solo and Plum Duo, based on the unique cassette technology in the company’s Plum 360 pump, deliver more accuracy in real-world conditions and eliminate the infusion consistencies found in traditional pumps. The company says they provide clinicians with predictable performance and reliable infusion documentation.