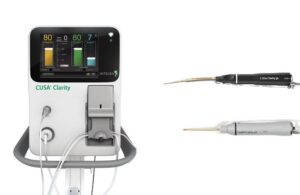
CUSA (Clarity Ultrasonic Surgical Aspirator) now has clearance for specific cardiac indications. Indications now encompass the debridement of unwanted tissue in cardiac surgeries. These include valve replacement and repair.
Princeton, New Jersey-based Integra said that, in addition to cardiac surgery, the FDA granted CUSA an indication for use in surgical procedures. These procedures include when fragmentation, emulsification and aspiration of soft and hard (bone) tissue is desirable. This extends CUSA’s uses to a range of surgeries beyond cardiac. Those include neurosurgery, plastic and reconstructive surgery, orthopedic surgery, thoracic surgery, laparoscopic surgery, gynecological surgery and liver resection and transplant surgery.