FDA clearance covers the treatment of venous in-stent restenosis (ISR) and native vessel obstructions. It comes just over a year after the company raised $13 million in its Series A funding round to support Recana.
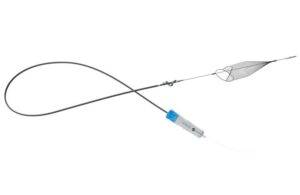
Redwood City, California-based InterVene designed Recana to recanalize and restore patency in chronically obstructed deep veins and venous stents. It particularly targets when recanalization capabilities are limited or when long-term chronic stent maintenance is necessary. Recana utilizes standard fluoroscopy/IVUS-guided interventional techniques. The system features a debulking catheter, introducer and collection sheaths and nitinol collection baskets.
InterVene says Recana’s stainless-steel helical coring element has a sharpened beveled edge. This enables it to clear tough, residual venous obstructions and occlusions, with its spiral nose cone assisting with crossing. The nitinol baskets deploy from the lower extremities to capture thrombotic material, streamlining the procedural workflow.