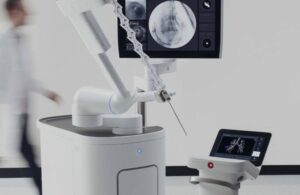
The Ion robotic bronchoscopy system — which the FDA cleared in 2019 — is a robotic-assisted, catheter-based platform. Ion enables minimally invasive biopsy deep within the lung. Fiber optic shape sensor technology along the catheter’s length provides its precise location and shape information throughout the procedure.
Ion’s ultra-thin, shape-sensing catheter can navigate deep into the lung to access small, hard-to-reach nodules. It enables physicians to precisely position biopsy tools and sample potentially cancerous tissue.