The approval, known as a CE Mark, allows the company’s InVera Infusion Device to be marketed and used across the European Union.
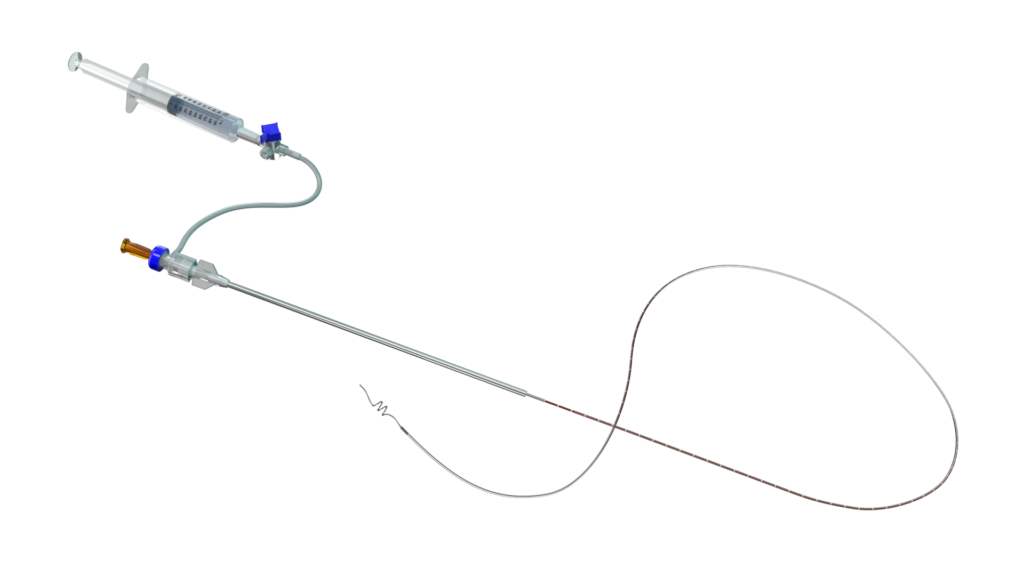
Unlike many existing technologies that burn the vein, the InVera device uses a non-thermal approach. The procedure is carried out in a doctor’s office, requires only a single injection of local anaesthetic, and does not need specialised hospital equipment. This represents a major step forward in expanding non-thermal options for physicians in the management of Chronic Venous Disease.
The device uses a thin catheter inserted into the vein under ultrasound guidance, a technique already familiar to many physicians, and is designed to enhance infusion of physician-specified agents more effectively inside the vein.
Chronic Venous Disease affects around one in four adults and can progress from visible varicose veins to painful leg ulcers if left untreated. Despite estimated to affect over 120 million people across Europe and the US, only around 1% of those living with venous disease currently receive interventional treatment each year, underlining a significant gap in care that InVera aims to address.