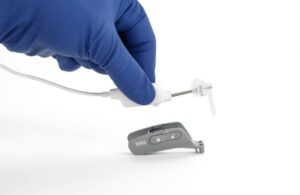
The iotaSoft insertion system enables precise and controlled electrode array insertion during cochlear implant surgery. St. Paul, Minnesota-based IotaMotion says it standardizes a critical, delicate step of this type of procedure. In doing so, the robotic-assisted technology helps to preserve delicate cochlear structures, the company says.
Now, iotaSoft has FDA clearance for use in patients four years of age and older. The clearance extends access to robotic-assisted cochlear implantation for school-aged children. Cincinnati Children’s Hospital became the first dedicated pediatric center to adopt the system. It joins more than 35 cochlear implant centers across the U.S.