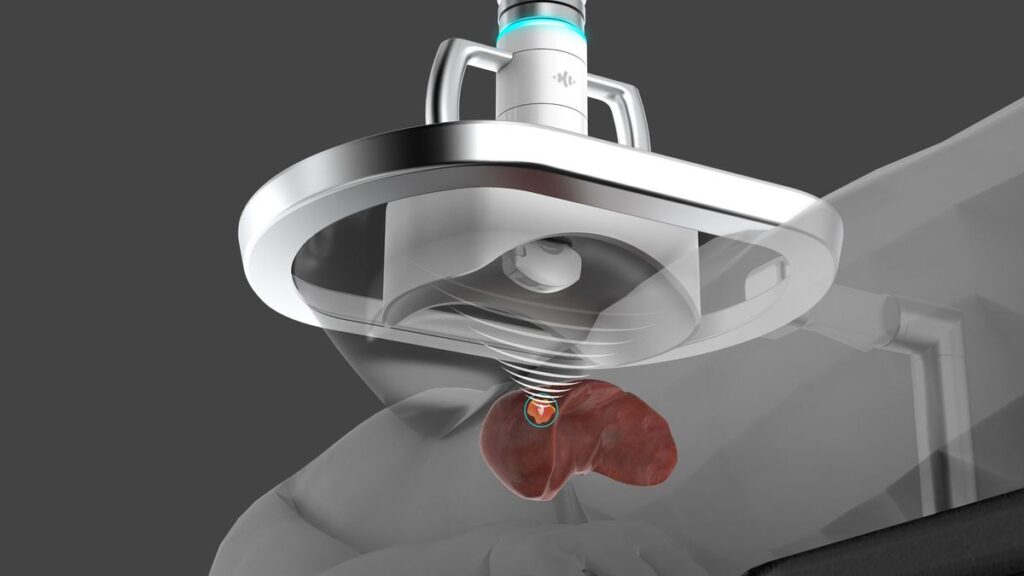
HistoSonics has received de novo authorization for a device that uses sound waves to destroy liver tumors, clearing it to sell the product in the U.S.
The company, which received investment from Johnson & Johnson last year, has developed the Edison system to offer a minimally invasive alternative to surgical resection and thermal ablation in patients with primary and metastatic liver cancers.
HistoSonics received the Food and Drug Administration authorization after linking the device to a technical success rate of 95.5% in a trial of patients with primary and secondary liver tumors.