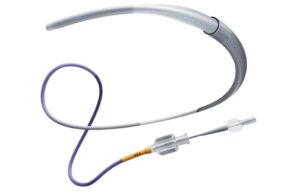
The launch comes just one day after media reports suggested that the company wants to sell its Cerenovus stroke business, the unit that makes the CereGlide system.
CereGlide 92, a next-generation .092″ catheter, features the InnerGlide 9 delivery aid. Its indication covers facilitating the insertion and guidance of interventional devices in the neurovascular system. The inner diameter catheter system allows physicians to achieve large distal access. Johnson & Johnson designed it to provide flow reduction in the M1 when inserting devices for the revascularization of patient with acute ischemic stroke.