According to the FDA 510(k) database, the agency received Jupiter Endovascular’s submission on June 30. It delivered the clearance on Sept. 12.
The company took to LinkedIn to announce the regulatory milestone:
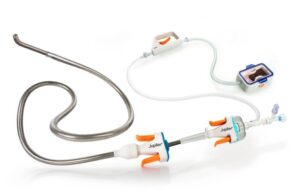
“FDA Cleared! Our Vertex catheter just received 510(k) clearance for the insertion of endovascular devices,” the company wrote. “Vertex is the first and only FDA-cleared intravascular device with Transforming Fixation (TFX) — able to shift from flexible to firm on demand, giving physicians true stability and control.”
The company also said it expects “even bigger” updates for the company this fall. (Read more about the Vertex catheter at Medical Design & Outsourcing.)
Jupiter’s Vertex catheter comprises part of the vertex system designed to treat acute pulmonary embolism (PE). The innovative endovascular procedure offers high levels of control and precision. Menlo Park, California-based Jupiter received FDA investigational device exemption for Vertex in August 2024. It announced the first cases with the system a month later.