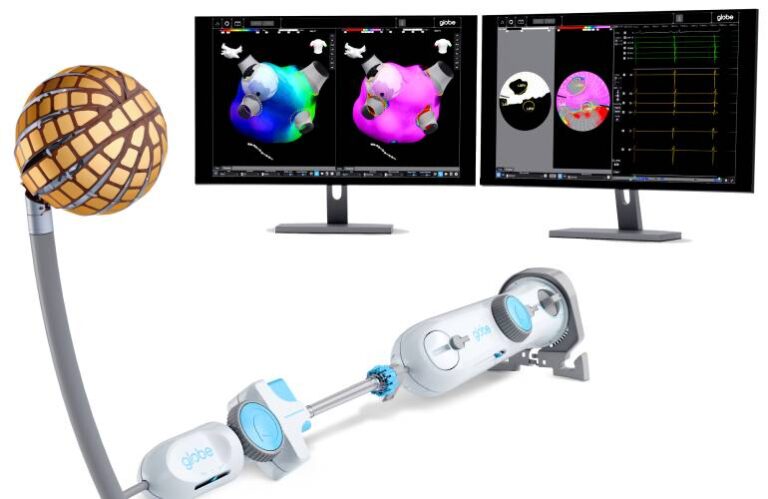
These mark the first cases with Globe, a treatment for AFib, after its recent approval by the FDA. Last month, the FDA granted Globe premarket approval (PMA), plus 510(k) clearance for the Globe introducer sheath and Globe pulsed field mapping software.
Dr. Devi Nair completed the inaugural U.S. procedures. Nair serves as chief of cardiac EP and EP research at St. Bernard’s Medical Center (Jonesboro, Arkansas).
“We are very excited to offer our patients access to this groundbreaking new technology,” said Nair. “The Globe pulsed field system gives me greater confidence that each lesion is delivered safely and effectively, to support better outcomes for my patients while also simplifying the procedure.”