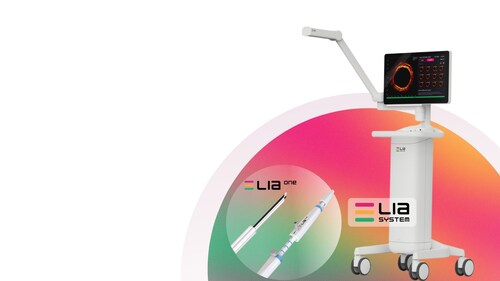
This milestone follows the recent U.S. Food and Drug Administration (FDA) 510(k) clearance of LEADOPTIK’s LIA™ System [link to FDA clearance] and represents an important step toward validating its potential to improve diagnostic accuracy during lung biopsy procedures.
The first-in-human case was performed using the LIA™ system’s integrated optical imaging, which provides real-time, depth-resolved insights at the point of tissue sampling. The technology is designed to help physicians confirm that biopsy tissue is collected from the intended target, addressing a longstanding challenge in lung cancer diagnosis.