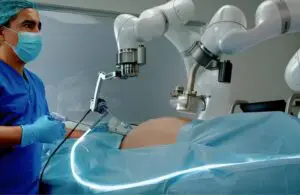
Levita Magnetics has received FDA approval for its MARS magnetic-assisted robotic surgery platform. The platform, designed for high-volume abdominal surgery, utilizes magnets and machines to minimize incisions and provide surgeons with enhanced control during laparoscopic procedures. The compact system can be easily integrated into existing operating rooms.
This development builds upon the success of Levita’s previous product, the Levita Magnetic Surgical System, and aims to offer patients less invasive surgery, faster recovery, and reduced scarring. Surgeons benefit from improved control over surgical instruments and the ability to retract large tissues and organs. The MARS system also enhances efficiency in hospitals and ambulatory surgery centers, leading to more effective deployment of personnel and improved patient procedures.
Levita Magnetics believes that MARS has the potential to revolutionize the surgical industry and contribute to advancements in medical innovation.