Clearance paves the way for the use of the TMS technology to treat patients with major depressive disorder (MDD), obsessive-compulsive disorder (OCD) and anxious depression.
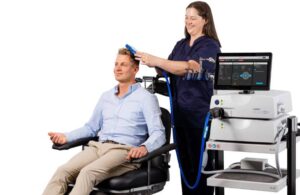
Minneapolis-based Magstim designed Horizon Inspire to address physician needs with high-power air cooling and the ability to deliver back-to-back customizable treatments. It offers ease of use, cost-effectiveness and portability between clinic rooms.
The company built Horizon Inspire on its Magstim TMS technology, which has prior FDA clearances. It leverages intuitive preset clinical workflows to simplify the treatment process. Magstim says its system delivers precise results with no pulse decay, ensuring the correct dosage. The air-cooled coil reduces downtime and eliminates additional cooling expenses, while advanced data analytics tools improve treatment efficacy.