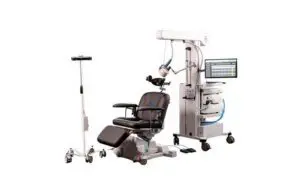
The latest platform builds on the previous H 3.0 system for treating patients with depressive or obsessive-compulsive conditions.
Horizon 3.0 with StimGuide Pro is the first integrated TMS system with navigation, Magstim said in a news release. It includes new advanced camera technology and software designed to allow for precise treatment targeting.
The system centralizes all patient treatments and technology on one screen to reduce complexity as well. It also has a Bluetooth anatomical pointer for mapping and treating patients.