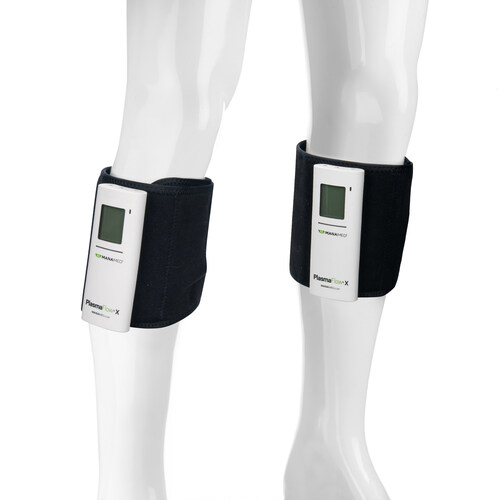
Designed from the inside out, PlasmaFlow® X delivers a compact design to help save shelf space in surgery centers and warehouses nationwide—while reducing packaging waste. With an all–day battery that offers double the battery life vs. the previous PlasmaFlow model, the latest controller architecture, and USB–C charging, it’s built for seamless, everyday use at home and across care settings. An expanded LCD presents the essentials instantly—battery charge status, pressure (mmHg), patient usage, and adherence—bringing a modern, intuitive experience to sequential compression. The low–profile, lightweight sleeve is engineered for comfort and a sleek fit for patients on the move.
“PlasmaFlow X is a generational leap for sequential compression,” said Trevor Theriot, President + CEO of ManaMed. “In a category long defined by incremental updates, our team re–imagined everything—a more compact design, all–day battery, USB–C convenience, and a big beautiful, informative display—to deliver industry leading therapy in a remarkably portable system. This is our most exciting product launch in ManaMed’s 10–year history, and it underscores our commitment to innovation that serves patients, clinicians, and partners.”