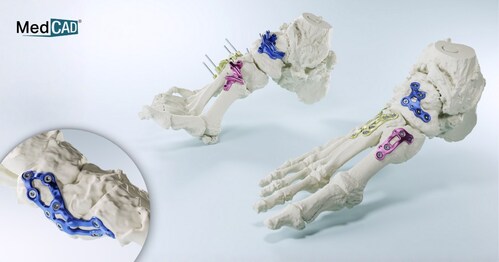
“This is the first and the only FDA-cleared, truly patient-specific 3D-printed titanium implant and cutting guide system solution in the U.S.,” said Nancy Hairston, CEO and president of MedCAD. “There are no other truly custom foot plates on the market, and no other truly custom comprehensive system like ours.”
The AccuStride system is designed to provide truly patient-specific custom solutions for revision procedures, deformities, arthritic or diabetic conditions, and trauma-related foot and ankle injuries. Components are constructed of UV-curable acrylate polymers or titanium alloy materials and can be delivered in as few as five days after receipt of medical imaging and surgeon design approval. The system is intended for use in patients 12 years and older.