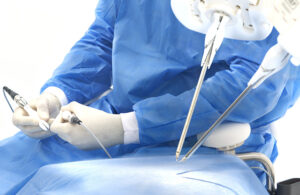
The FDA granted the IDE for a clinical study evaluating microsurgery intervention for Alzheimer’s disease. The REMIND (robotic-enabled microsurgical intervention for neurodegenerative disease) study will collect safety and effectiveness data for the Symani surgical robot system and microsurgical techniques. It evaluates Symani in reestablishing lymphatic drainage pathways in the deep cervical lymph nodes (dCLNs). Subjects have Alzheimer’s and confirmed lymphatic obstruction.
Symani provides advanced solutions for a range of open surgeries. That includes post-mastectomy breast cancer reconstruction, extremity reconstruction using free tissue transfer and lymphatic system repair. The company’s tiny NanoWrist instruments help to access and suture small, delicate anatomies.