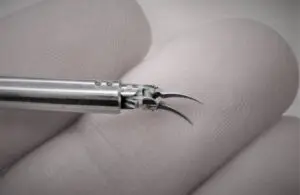
The FDA cleared the company’s NanoWrist Scissors and Forceps for soft tissue dissection. Clearance enables delicate tissue manipulation and dissection in robotic microsurgery. It expands the capabilities of the company’s Symani surgical robot beyond anastomosis, including critical steps in complex surgery.
The company’s Symani surgical robotic system allows surgeons to connect lymphatic vessels to nearby veins. By connecting the vessels, smaller than several strands of hair, the procedure helps reroute fluid and relieve swelling. Medical Microinstruments touts Symani as the only microsurgical robot approved for commercial use. It won FDA de novo clearance for Symani, covering soft tissue manipulation to perform microsurgery, in April 2024.