The company said clearance makes Cranial Navigation the world’s first AR system cleared for intraoperative guidance in cranial neurosurgery. It marks the company’s second major FDA clearance this year, following the launch of its Spine Navigation platform.
New York-based Medivis’ FDA milestones go as far back as 2019, when the agency cleared its SurgicalAR platform. At that time, the company also touted strategic partnerships with Verizon and Microsoft.
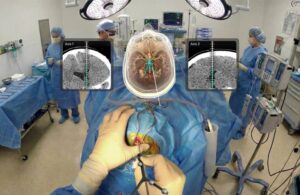
The Cranial Navigation platform uses AR to spatially map patient imaging within the operative field. Medivis said it gives surgeons a clear, real-time view of critical anatomy and planned trajectories. It designed the approach to support faster, more confident decision-making during cranial procedures. The platform can also minimize workflow disruption and reduce dependence on external monitors.