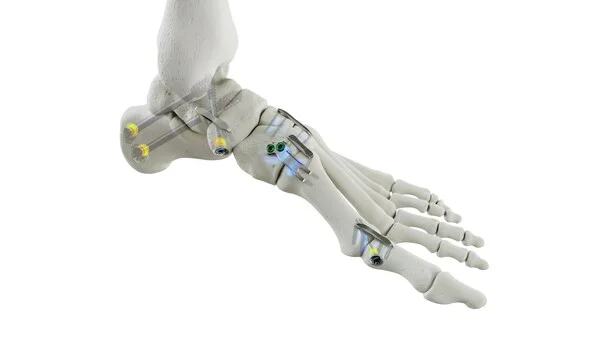
Medline UNITE, a leader in the foot and ankle surgery market, announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its latest implant family, the REFLEX® HYBRID Nitinol Implant System.
While nitinol staples have steadily risen in popularity, static plates and screws are widely used in conjunction with staples to provide added fixation and stability. A key disadvantage of such hybrid constructs is the inherent neutralization of compression caused by the addition of static devices such as locking plates and cannulated screws. REFLEX HYBRID combines the compression of a nitinol staple with the stability of a locking plate, offering indication-specific implants designed for MTP fusions and Lapidus procedures. When paired with a cannulated screw with a Dynamic Disc, or a REFLEX nitinol staple, the construct provides dynamic biplanar compression and fixation for a fully dynamic construct.
“REFLEX HYBRID further demonstrates our commitment to offering innovative solutions for foot and ankle surgeons. The first to market product addresses gaps in the current competitive landscape, including offering indication specific designs, intraoperative compression, and intraoperative adjustment with a nitinol implant,” said Scott Goldstein, vice president of product management for Medline UNITE.