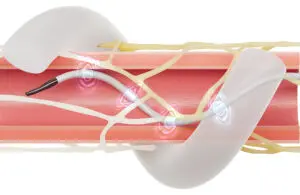
This process allows the agency to review and develop a national Medicare coverage policy for RDN procedures. CMS initiated the analysis after Medtronic requested beneficiary access support for its Symplicity Spyral RDN system.
A national coverage policy would also benefit the Recor Medical Paradise RDN system. Recor Medical received an FDA nod for Paradise in November 2023. Medtronic the second company with such approval, receiving its nod just weeks later. Both companies preivously praised CMS New Technology Add-on Payment (NTAP) program acceptance for their technologies in August.
CMS’ analysis follows Medtronic’s effort to pilot framework for the Transitional Coverage for Emerging Technologies (TCET) pathway. This would establish coverage for Symplicity Spyral. The medtech giant said CMS expects to complete its analysis on Oct. 11, 2025. Until a determination goes into effect, the company said Symplicity Spyral procedures will continue to be evaluated for coverage based on medical necessity for individual Medicare patients.