DUBLIN, March 27, 2024 /PRNewswire/ — Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced that the United States Food and Drug Administration (FDA) has approved the Evolut™ FX+ transcatheter aortic valve replacement (TAVR) system for the treatment of symptomatic severe aortic stenosis. The latest Evolut FX+ TAVR system maintains the valve performance benefits of the legacy Evolut TAVR platform and is designed to facilitate coronary access.
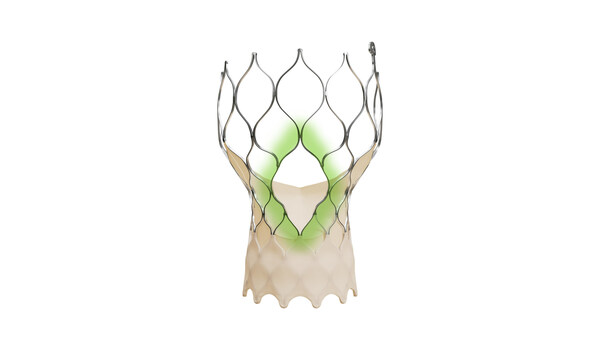
The Medtronic Evolut™ FX+ TAVR system leverages market-leading valve performance with the addition of larger windows to facilitate coronary access.
The Medtronic Evolut™ FX+ TAVR system leverages market-leading valve performance with the addition of larger windows to facilitate coronary access.
The Evolut FX+ TAVR system offers larger coronary access windows through a modified diamond-shaped frame design, which is four times larger than previous iterations of the Evolut TAVR system. Evolut FX+ provides increased space for catheter maneuverability to facilitate access to coronary arteries of varying patient anatomies. Additionally, the new design does not compromise the market leading valve performance, excellent hemodynamics, and radial strength that clinicians expect from the Evolut platform.