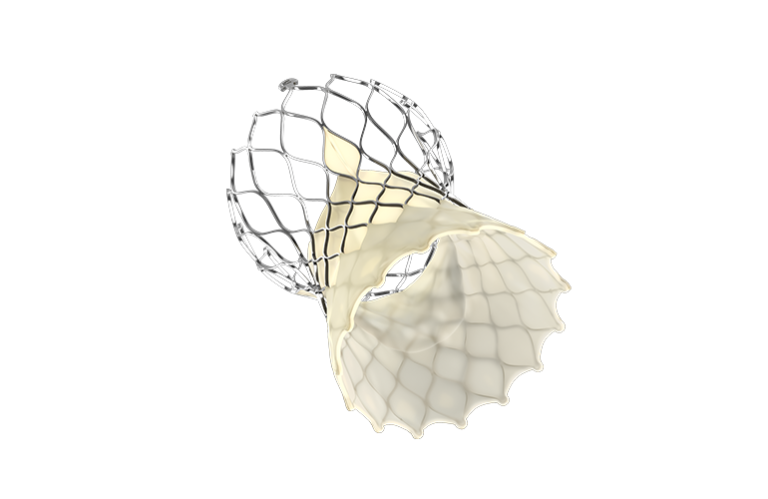
The approval enables the implantation of a new Evolut transcatheter aortic valve inside any previously implanted, failed valve. The expanded indication applies to patients with severe aortic stenosis who are considered high-risk for open heart surgery.
“The CE Mark approval for the Evolut TAVI system’s redo TAVI procedure is great news for physicians working in this field, but most importantly for patients with failing transcatheter heart valves, who now have a crucial new treatment option,” said Dan Blackman, consultant interventional cardiologist at Leeds Teaching Hospitals NHS Trust, United Kingdom. “This minimally invasive procedure not only offers an alternative for patients at high risk for surgery but also underscores the commitment to improving outcomes and extending the benefits of TAVI therapy.”