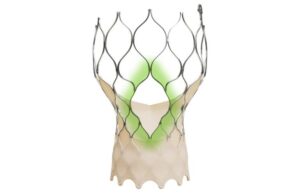
This allows for the implantation of a new Evolut transcatheter aortic valve replacement (TAVR) system inside any failed previously implanted TAV. It comes on the heels of CE mark for the Evolut Pro+, FX and FX+ systems for this indication.
Redo-TAVR is indicated for patients experiencing failure of any TAV. That includes severe aortic stenosis patients at high risk for open-heart surgery.
In addition to the approval, Medtronic also launched the RESTORE study. It evaluates the outcomes of redo TAVR in patients experiencing symptomatic bioprosthetic valve failure.